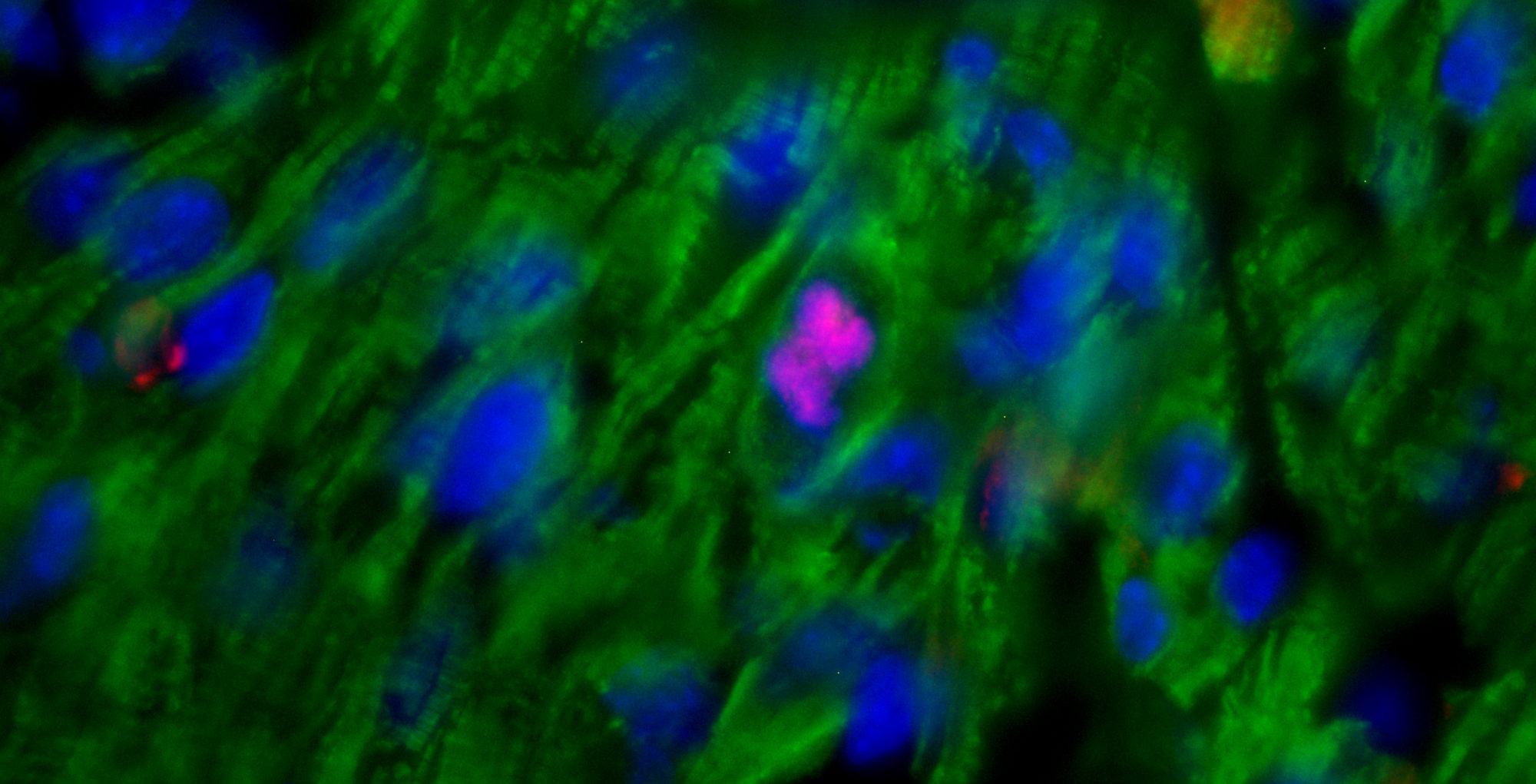
Hematopoietic stem and progenitor cells (HSPCs) are regulated by various bone marrow stromal cell types. Here we identified rare activated bone marrow monocytes and macrophages with high expression of α-smooth muscle actin (α-SMA) and the cyclooxygenase COX-2 that were adjacent to primitive HSPCs. These myeloid cells resisted radiation-induced cell death and further upregulated COX-2 expression under stress conditions. COX-2-derived prostaglandin E(2) (PGE(2)) prevented HSPC exhaustion by limiting the production of reactive oxygen species (ROS) via inhibition of the kinase Akt and higher stromal-cell expression of the chemokine CXCL12, which is essential for stem-cell quiescence. Our study identifies a previously unknown subset of α-SMA(+) activated monocytes and macrophages that maintain HSPCs and protect them from exhaustion during alarm situations.
Vai all’articolo originale (in inglese): Aya Ludin, Tomer Itkin, Shiri Gur-Cohen, Alexander Mildner, Elias Shezen, Karin Golan, Orit Kollet, Alexander Kalinkovich, Ziv Porat, Gabriele D’Uva, Amir Schajnovitz, Elena Voronov, David A Brenner, Ron N Apte, Steffen Jung, Tsvee Lapidot. Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nature Immunology, 2012

 Abbiamo sviluppato Bitnos.com, un sistema operativo libero e basato sul web che permette di utilizzare le migliori applicazioni online, motori di ricerca e siti web gratuiti in campo biomedico. Con applicazioni e servizi on-line (noti anche come applicazioni web o webware), non è necessario scaricare e installare nulla. Tutti i servizi saranno direttamente disponibili per te in un solo click. Queste applicazioni e servizi funzionano indipendentemente dal sistema operativo che si sta utilizzando, in esecuzione tramite il browser come client. Hai solo bisogno di accedervi on-line.
Abbiamo sviluppato Bitnos.com, un sistema operativo libero e basato sul web che permette di utilizzare le migliori applicazioni online, motori di ricerca e siti web gratuiti in campo biomedico. Con applicazioni e servizi on-line (noti anche come applicazioni web o webware), non è necessario scaricare e installare nulla. Tutti i servizi saranno direttamente disponibili per te in un solo click. Queste applicazioni e servizi funzionano indipendentemente dal sistema operativo che si sta utilizzando, in esecuzione tramite il browser come client. Hai solo bisogno di accedervi on-line.