La nostra ricerca su meccanismi post-trascrizionali in cellule staminali tumorali è stata pubblicata!
Hypoxia has been long-time acknowledged as major cancer-promoting microenvironment. In such an energy-restrictive condition, post-transcriptional mechanisms gain importance over the energy-expensive gene transcription machinery. Here we show that the onset of hypoxia-induced cancer stem cell features requires the beta-catenin-dependent post-transcriptional up-regulation of CA9 and SNAI2 gene expression. In response to hypoxia, beta-catenin moves from the plasma membrane to the cytoplasm where it binds and stabilizes SNAI2 and CA9 mRNAs, in cooperation with the mRNA stabilizing protein HuR. We also provide evidence that the post-transcriptional activity of cytoplasmic beta-catenin operates under normoxia in basal-like/triple-negative breast cancer cells, where the beta-catenin knockdown suppresses the stem cell phenotype in vitro and tumor growth in vivo. In such cells, we unravel the generalized involvement of the beta-catenin-driven machinery in the stabilization of EGF-induced mRNAs, including the cancer stem cell regulator IL6. Our study highlights the crucial role of post-transcriptional mechanisms in the maintenance/acquisition of cancer stem cell features and suggests that the hindrance of cytoplasmic beta-catenin function may represent an unprecedented strategy for targeting breast cancer stem/basal-like cells.

Vai all’articolo originale (in inglese): Gabriele D’Uva*, Sara Bertoni, Mattia Lauriola, Sabrina De Carolis, Annalisa Pacilli, Laura D’Anello, Donatella Santini, Mario Taffurelli, Claudio Ceccarelli, Yosef Yarden, Lorenzo Montanaro, Massimiliano Bonafé, Gianluca Storci. Beta-Catenin/HuR Post-Transcriptional Machinery Governs Cancer Stem Cell Features in Response to Hypoxia. PloS One, 2013 (co-corresponding author)
EMBO Meeting 2013! Parleremo del nostro progetto sulla rigenerazione cardiaca!
Siamo stati selezionati per la presentazione orale del nostro progetto intitolato “Heart regeneration and tumorigenicity: oncogenic ErbB2 as a driving force” alla conferenza EMBO meeting “Cardiac Biology: From Development to Regenerative Medicine” (7-10 Giugno 2013, EMBL Heidelberg, Germania).



Il nostro articolo è su JCI! Siamo felici di aver contribuito a questo progetto internazionale sull’uscita fisiologica delle cellule staminali ematopoietiche!
Regulation of hematopoietic stem and progenitor cell (HSPC) steady-state egress from the bone marrow (BM) to the circulation is poorly understood. While glycogen synthase kinase-3β (GSK3β) is known to participate in HSPC proliferation, we revealed an unexpected role in the preferential regulation of CXCL12-induced migration and steady-state egress of murine HSPCs, including long-term repopulating HSCs, over mature leukocytes. HSPC egress, regulated by circadian rhythms of CXCL12 and CXCR4 levels, correlated with dynamic expression of GSK3β in the BM. Nevertheless, GSK3β signaling was CXCL12/CXCR4 independent, suggesting that synchronization of both pathways is required for HSPC motility. Chemotaxis of HSPCs expressing higher levels of GSK3β compared with mature cells was selectively enhanced by stem cell factor-induced activation of GSK3β. Moreover, HSPC motility was regulated by norepinephrine and insulin-like growth factor-1 (IGF-1), which increased or reduced, respectively, GSK3β expression in BM HSPCs and their subsequent egress. Mechanistically, GSK3β signaling promoted preferential HSPC migration by regulating actin rearrangement and microtubuli turnover, including CXCL12-induced actin polarization and polymerization. Our study identifies a previously unknown role for GSK3β in physiological HSPC motility, dictating an active, rather than a passive, nature for homeostatic egress from the BM reservoir to the blood circulation.
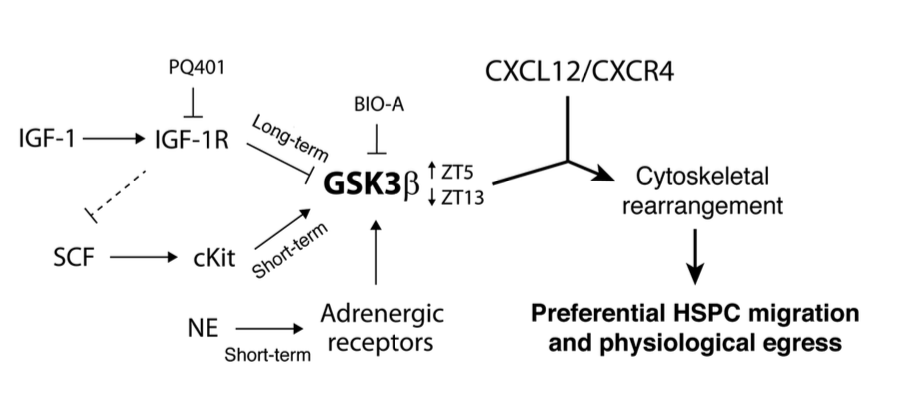
Vai all’articolo originale (in inglese): Kfir Lapid, Tomer Itkin, Gabriele D’Uva, Yossi Ovadya, Aya Ludin, Giulia Caglio, Alexander Kalinkovich, Karin Golan, Ziv Porat, Massimo Zollo, Tsvee Lapidot. GSK3β regulates physiological migration of stem/progenitor cells via cytoskeletal rearrangement. The Journal of clinical investigation, 2013