We are excited to announce our new publication in Nature Cardiovascular Research, in which we describe an innovative strategy to enhance heart regeneration through blockade of the glucocorticoid receptor.
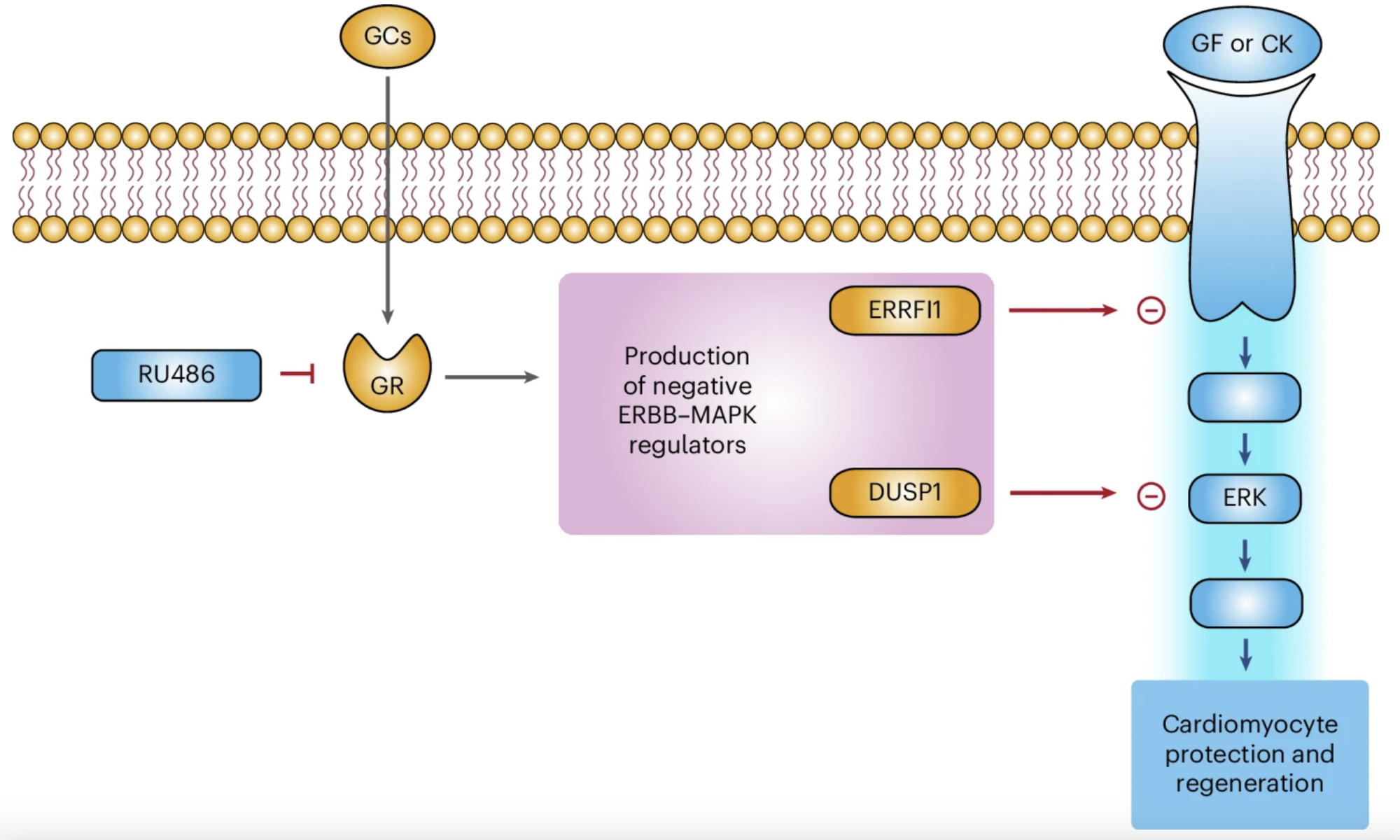
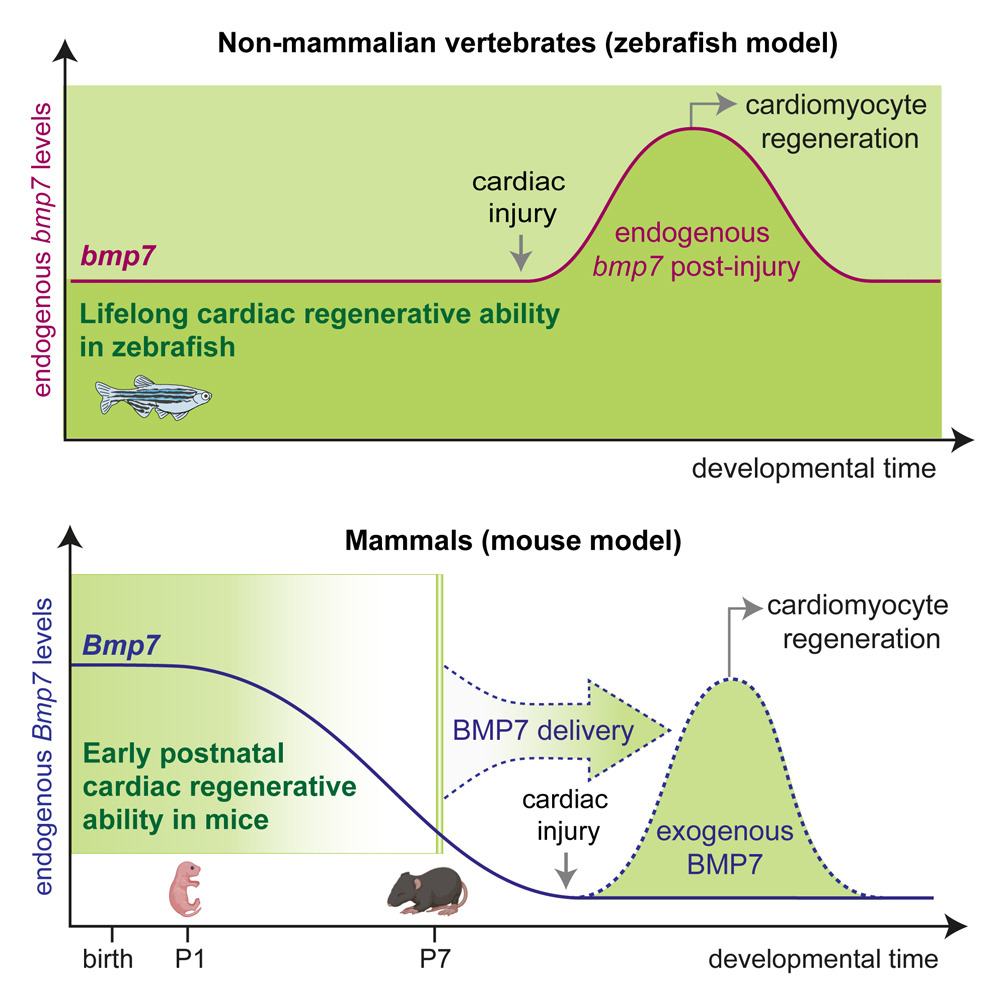
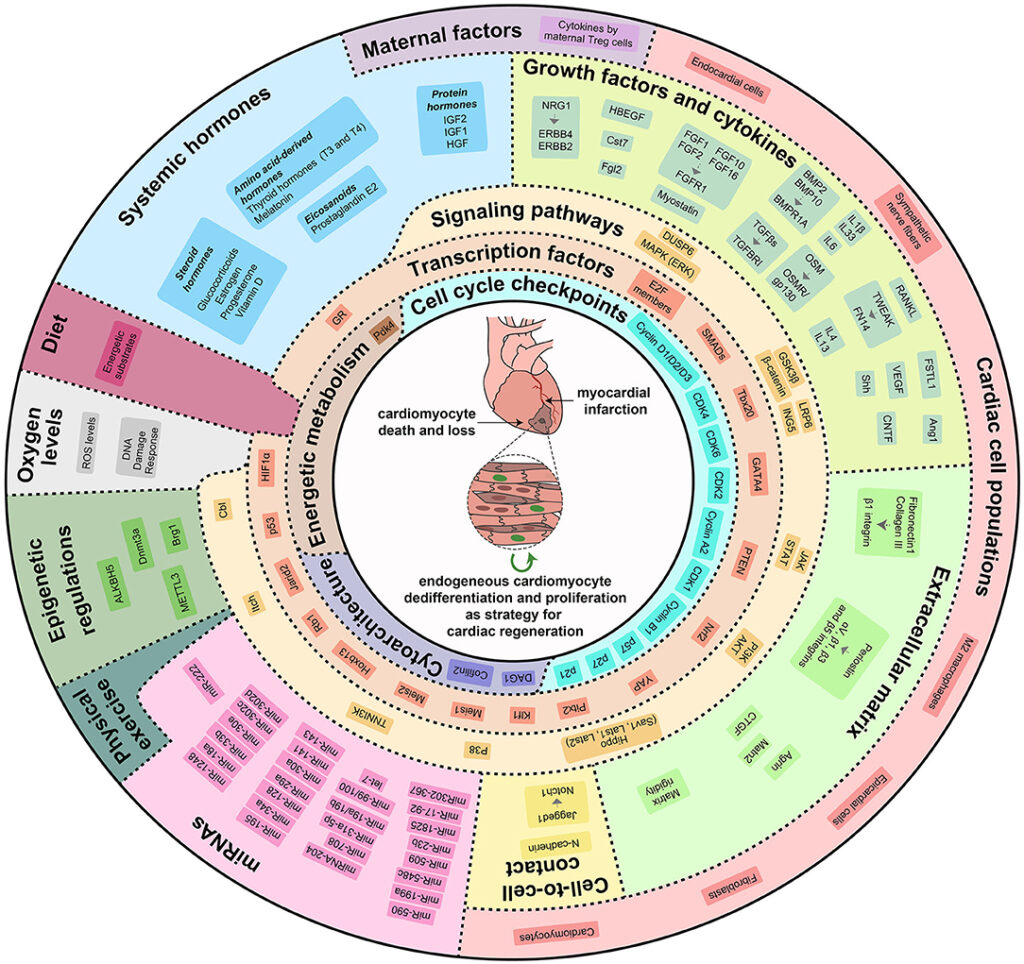
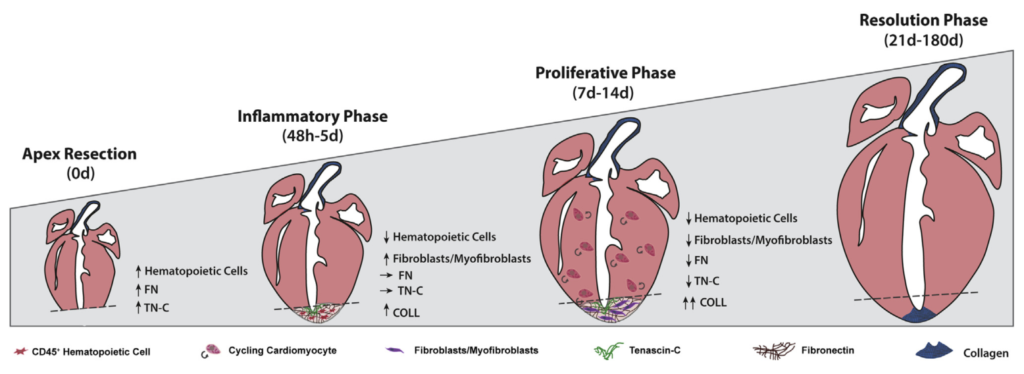
In our study, we demonstrate that glucocorticoids—steroid hormones that are physiologically present in the circulation—play a key role in limiting the responsiveness of cardiomyocytes to major regenerative growth factors and cytokines. Specifically, we show that these hormones act as a true hormonal brake, contributing to the loss of the heart’s regenerative capacity during late postnatal stages and in adulthood.
A central finding of our work is the demonstration that pharmacological blockade of the glucocorticoid receptor can remove this brake, restoring the ability of cardiomyocytes to respond to proliferative stimuli. This approach substantially enhances the effectiveness of growth factor–based regenerative therapies, even in mature hearts.
In preclinical models, we further observed that the combination of a glucocorticoid receptor antagonist with a regenerative factor produces markedly superior results compared with single treatments. This effect is particularly relevant in settings of cardiac damage associated with anthracycline-based cancer therapies, where the combined approach improved cardiomyocyte survival and preserved cardiac function.
Overall, we believe that these findings open new avenues for the development of combined therapeutic strategies aimed at regenerating cardiac tissue and that, if clinically validated, they could have a significant impact on the treatment of heart failure.
Congratulations to Silvia Da Pra and Stefano Boriati, a postdoctoral researcher and a PhD student in our group, who carried out the majority of the experimental work, and thanks to all team members Carmen Miano, Francesca Sacchi, Chiara Bongiovanni, Irene Del Bono and Nicola Pianca for their contributions. We also thank the collaborators who were instrumental to the success of this project, in particular the research groups led by Eldad Tzahor (Weizmann Institute of Science, Israel), Catherine Wilson (University of Cambridge, United Kingdom), and Mattia Lauriola and Carlo Ventura (University of Bologna, Italy).
Go to the full article: : Da Pra S, Boriati S, Miano M, Sacchi F, Batho C, Bongiovanni C, Del Bono I, Aharonov A, Pianca N, Tassinari R, Dahir R, Ventura C, Lauriola M, Tzahor E, Wilson C & D’Uva G. Harnessing glucocorticoid receptor antagonism to enhance the efficacy of cardiac regenerative growth factors and cytokines : Nature Cardiovascular Research 2026
Read-only access to the article (no subscription required): https://rdcu.be/e2EN0

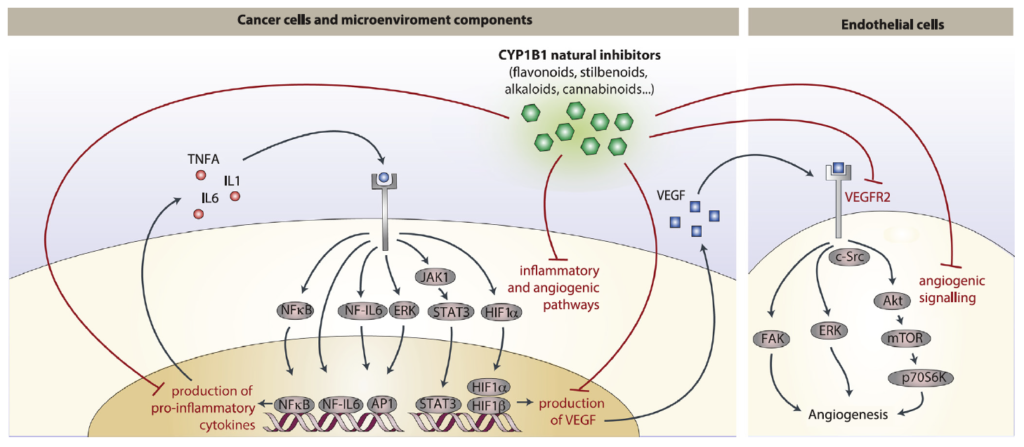 Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.
Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.