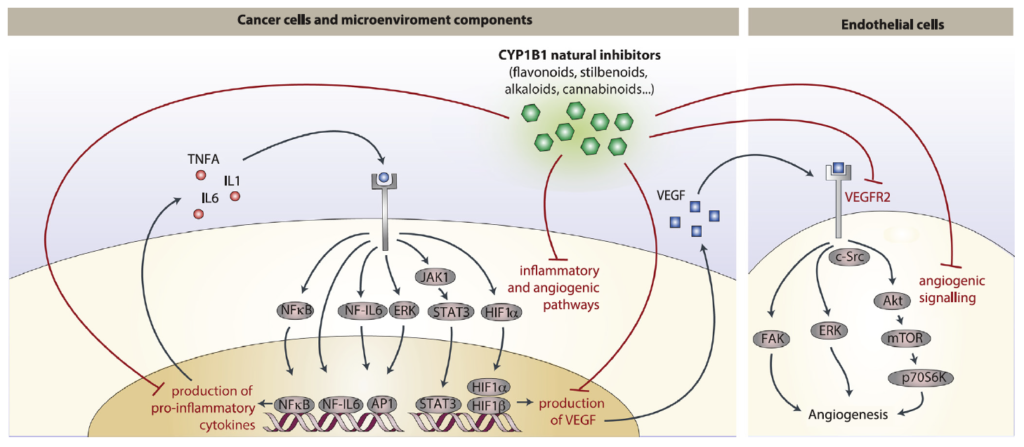
 Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.
Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.
Highlights:
•CYP1B1 triggers carcinogenesis by activating exogenous and endogenous molecules to reactive species.
•Other CYP1 family members play protecting roles against tumor formation by detoxifying carcinogenic compounds.
•CYP1B1 regulates multiple cell types within the cancer microenvironment, supporting a loop of inflammatory angiogenesis.
•Several chemopreventive phytochemicals are potent and specific CYP1B1 inhibitors.
•Specific CYP1B1 inhibition may translate into clinical chemoprevention/angioprevention approaches.
Go to the full article: D’Uva G, Baci D, Albini A and Noonan DM. Cancer chemoprevention revisited: Cytochrome P450 family 1B1 as a target in the tumor and the microenvironment. Cancer Treatment Reviews, 2017

 Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.
Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.