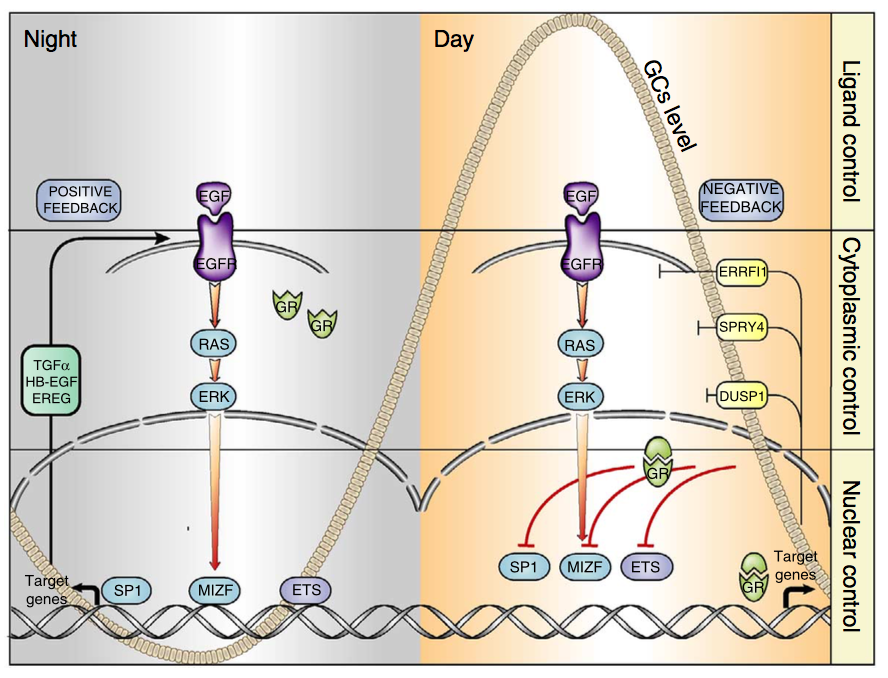
Signal transduction by receptor tyrosine kinases (RTKs) and nuclear receptors for steroid hormones is essential for body homeostasis, but the cross-talk between these receptor families is poorly understood. We observed that glucocorticoids inhibit signalling downstream of EGFR, an RTK. The underlying mechanism entails suppression of EGFR’s positive feedback loops and simultaneous triggering of negative feedback loops that normally restrain EGFR. Our studies in mice reveal that the regulation of EGFR’s feedback loops by glucocorticoids translates to circadian control of EGFR signalling: EGFR signals are suppressed by high glucocorticoids during the active phase (night-time in rodents), while EGFR signals are enhanced during the resting phase. Consistent with this pattern, treatment of animals bearing EGFR-driven tumours with a specific kinase inhibitor was more effective if administered during the resting phase of the day, when glucocorticoids are low. These findings support a circadian clock-based paradigm in cancer therapy.
Go to the full article: Mattia Lauriola, Yehoshua Enuka, Amit Zeisel, Gabriele D’Uva, Lee Roth, Michal Sharon-Sevilla, Moshit Lindzen, Kirti Sharma, Nava Nevo, Morris Feldman, Silvia Carvalho, Hadas Cohen-Dvashi, Merav Kedmi, Nir Ben-Chetrit, Alon Chen, Rossella Solmi, Stefan Wiemann, Fernando Schmitt, Eytan Domany & Yosef Yarden. Diurnal suppression of EGFR signalling by glucocorticoids and implications for tumour progression and treatment. Nature Communications, 2015
MEDIA COVERAGE
06.10.2014 – Science Daily Tumors might grow faster at nigh
06.10.2014 – NATURE WORLD NEWS: Is Cancer Growth Nocturnal?
06.10.2014 – Softpedia: Cancer Tumors Appear to Grow Faster and Spread More Easily at Night
06.10.2014 – Medical Xpress: Tumors might grow faster at night: Hormone that keeps us alert also suppresses the spread of cancer
06.10.2014 – Senior Journal: Cancer Grows at Night, Maybe That’s When to Attack, New Study Says
07.10.2014 – Daily Mail: Cancerous tumours ‘grow faster at night’ – and drugs to fight the disease might work better during sleep, study finds
07.10.2014 – Counsel&Heal: Cancer Treatment More Efficient During Night Time:Study
07.10.2014 – Science 2.0: Cancer Might Grow Faster At Night
07.10.2014 – The Health Site: Revealed — cancer spreads during nights
07.10.2014 – bhataramedia: Tumor Dapat Tumbuh Lebih Cepat Di Malam Hari
07.10.2014 – Jews News: Israeli study: Tumors might grow more quickly at night
08.10.2014 – The Times of Israel: Tumors may grow faster at night, Israeli study shows
08.10.2014 – Journal de la Science: Les tumeurs grossiraient plus vite la nuit
08.10.2014 – Haaretz: Night time may be the right time to treat cancer, find Israeli scientists
09.10.2014 – Weizmann USA: Why Cancer Drugs May Work Better While You Sleep
09.10.2014 – Nature子刊:夜间癌细胞扩散的更快?
09.10.2014 – TIME: Why Cancer Drugs May Work Better While You Sleep
09-10-2014 – Medisite: CANCER : LES MÉDICAMENTS PLUS EFFICACES LA NUIT
10.10.2014 – TopSante: Cancer : les tumeurs se développent plus vite la nuit
10.10.2014 – Forbes: Cancer May Grow Faster While We Sleep
10.10.2014 – Jerusalem Post Tumors may grow faster at night, say Weizmann scientists
10.10.2014 – Medcenter: Una hormona que nos mantiene alerta también suprime la diseminación del cáncer
16.10.2014 – EACR (European Association for Cancer Research): TUMOURS MIGHT GROW FASTER AT NIGHT
16.10.2014 – Revista Genetica Medica: Ritmos circadianos y tratamiento contra el cáncer
17.10.2014 – University Herald: Hormone Active During Day Supresses Growth of Cancer Cells, Study
20.10.2014 – Stato Quotidiano: Ricercatrice Manfredonia: “tumori crescono più in fretta di notte”
24.10.2014 – Huffington Post: Does Cancer Grow More Aggressively at Night?