Glad we could be part of this international project on heart development! Congratulations to Gonzalo del Monte Nieto and Richard Harvey!
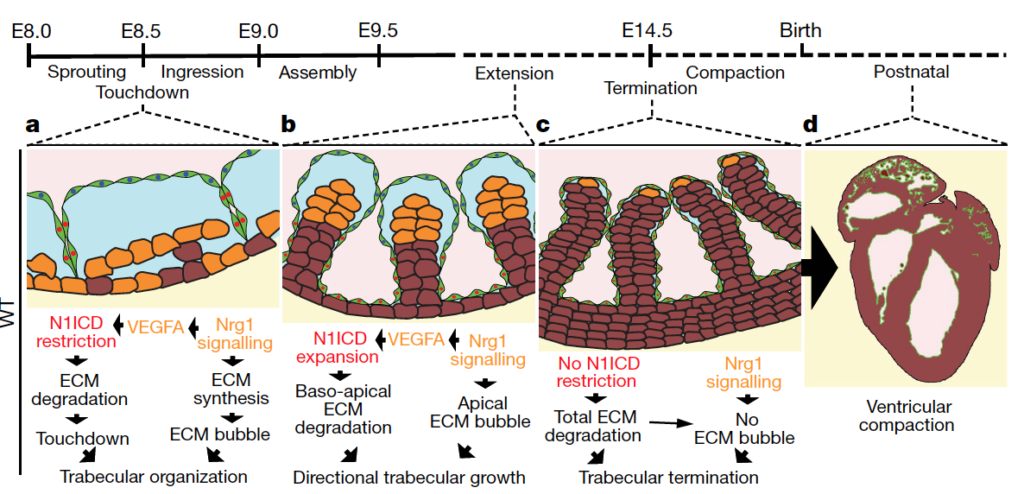
Abstract: In vertebrate hearts, the ventricular trabecular myocardium develops as a sponge-like network of cardiomyocytes that is critical for contraction and conduction, ventricular septation, papillary muscle formation and wall thickening through the process of compaction. Defective trabeculation leads to embryonic lethality or non-compaction cardiomyopathy (NCC). There are divergent views on when and how trabeculation is initiated in different species. In zebrafish, trabecular cardiomyocytes extrude from compact myocardium, whereas in chicks, chamber wall thickening occurs before overt trabeculation. In mice, the onset of trabeculation has not been described, but is proposed to begin at embryonic day 9.0, when cardiomyocytes form radially oriented ribs. Endocardium–myocardium communication is essential for trabeculation, and numerous signalling pathways have been identified, including Notch and Neuregulin (NRG). Late disruption of the Notch pathway causes NCC5. Whereas it has been shown that mutations in the extracellular matrix (ECM) genes Has2 and Vcan prevent the formation of trabeculae in mice and the matrix metalloprotease ADAMTS1 promotes trabecular termination, the pathways involved in ECM dynamics and the molecular regulation of trabeculation during its early phases remain unexplored. Here we present a model of trabeculation in mice that integrates dynamic endocardial and myocardial cell behaviours and ECM remodelling, and reveal new epistatic relationships between the involved signalling pathways. NOTCH1 signalling promotes ECM degradation during the formation of endocardial projections that are critical for individualization of trabecular units, whereas NRG1 promotes myocardial ECM synthesis, which is necessary for trabecular rearrangement and growth. These systems interconnect through NRG1 control of Vegfa, but act antagonistically to establish trabecular architecture. These insights enabled the prediction of persistent ECM and cardiomyocyte growth in a mouse NCC model, providing new insights into the pathophysiology of congenital heart disease.
Go to the full article: Del Monte-Nieto G, Ramialison M, Adam AAS, Wu B, Aharonov A, D’Uva G, Bourke LM, Pitulescu ME, Chen H, de la Pompa JL, Shou W, Adams RH, Harten SK, Tzahor E, Zhou B and Harvey RP. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature, 2018