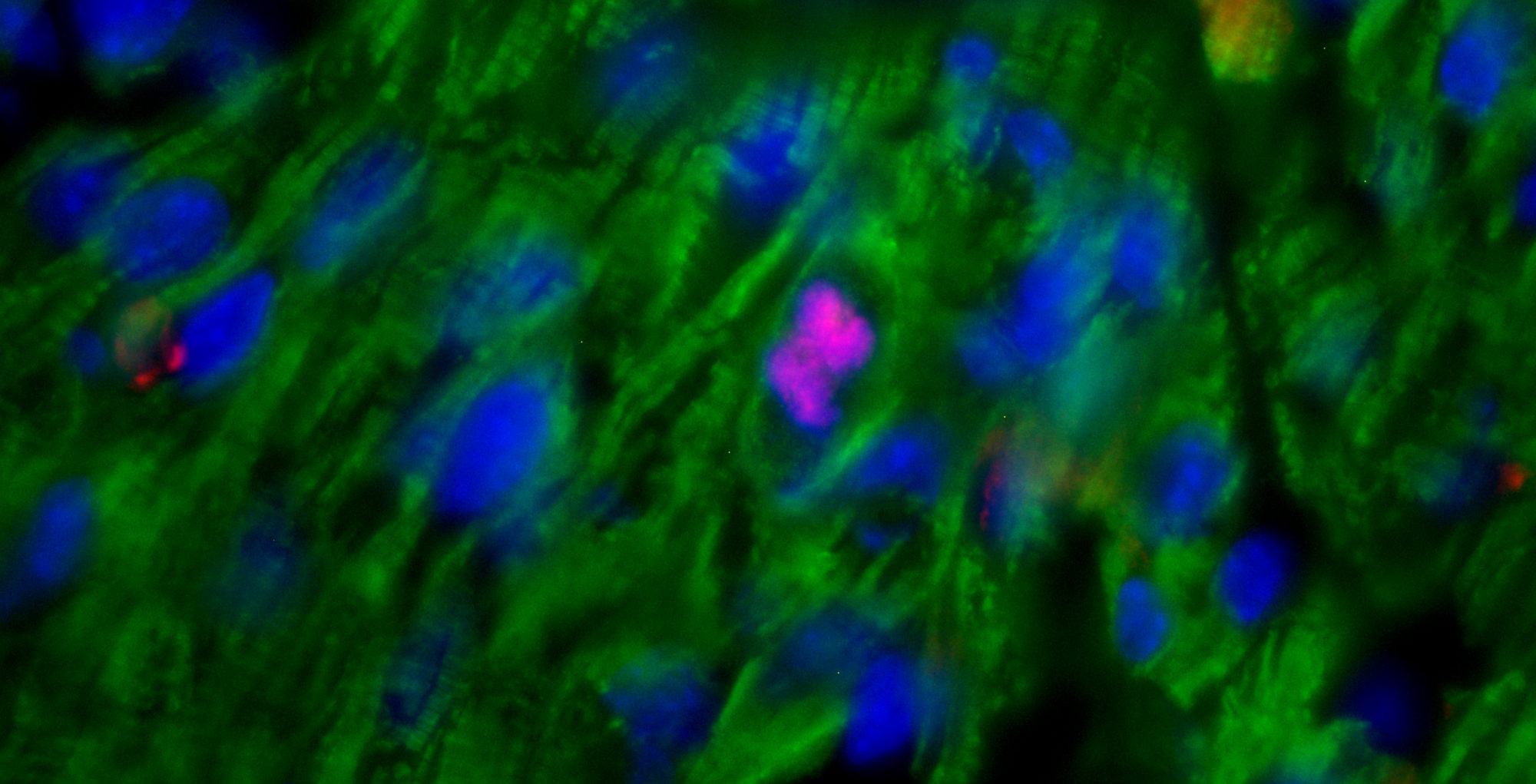
Hematopoietic stem and progenitor cells (HSPCs) are regulated by various bone marrow stromal cell types. Here we identified rare activated bone marrow monocytes and macrophages with high expression of α-smooth muscle actin (α-SMA) and the cyclooxygenase COX-2 that were adjacent to primitive HSPCs. These myeloid cells resisted radiation-induced cell death and further upregulated COX-2 expression under stress conditions. COX-2-derived prostaglandin E(2) (PGE(2)) prevented HSPC exhaustion by limiting the production of reactive oxygen species (ROS) via inhibition of the kinase Akt and higher stromal-cell expression of the chemokine CXCL12, which is essential for stem-cell quiescence. Our study identifies a previously unknown subset of α-SMA(+) activated monocytes and macrophages that maintain HSPCs and protect them from exhaustion during alarm situations.
Go to the full article: Aya Ludin, Tomer Itkin, Shiri Gur-Cohen, Alexander Mildner, Elias Shezen, Karin Golan, Orit Kollet, Alexander Kalinkovich, Ziv Porat, Gabriele D’Uva, Amir Schajnovitz, Elena Voronov, David A Brenner, Ron N Apte, Steffen Jung, Tsvee Lapidot. Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nature Immunology, 2012

 We developed Bitnos.com, a free, web-based, collaborative Operating System that provides you all the best free online biomedical applications, search engines and websites. With online applications and services (also known as web applications or webware), you do not need to download and install anything. All the services will be directly available for you in one click. These applications and services are cross-platform, running via your browser as a client irrespective of what operating system you are using. You just need to access them online.
We developed Bitnos.com, a free, web-based, collaborative Operating System that provides you all the best free online biomedical applications, search engines and websites. With online applications and services (also known as web applications or webware), you do not need to download and install anything. All the services will be directly available for you in one click. These applications and services are cross-platform, running via your browser as a client irrespective of what operating system you are using. You just need to access them online.