Popular Science: “Israel, where technology is reality”

Cardiac regeneration, cancer progression and cardiotoxicity of anticancer therapies
Popular Science: “Israel, where technology is reality”

 We were selected for the oral presentation of our project entitled “ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation” at The 5th Young Investigators Stem Cell Meeting, organized by Israeli Stem Cells Society (ISCS) (December 30, 2014, Technion, Haifa, Israel).
We were selected for the oral presentation of our project entitled “ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation” at The 5th Young Investigators Stem Cell Meeting, organized by Israeli Stem Cells Society (ISCS) (December 30, 2014, Technion, Haifa, Israel).
Signal transduction by receptor tyrosine kinases (RTKs) and nuclear receptors for steroid hormones is essential for body homeostasis, but the cross-talk between these receptor families is poorly understood. We observed that glucocorticoids inhibit signalling downstream of EGFR, an RTK. The underlying mechanism entails suppression of EGFR’s positive feedback loops and simultaneous triggering of negative feedback loops that normally restrain EGFR. Our studies in mice reveal that the regulation of EGFR’s feedback loops by glucocorticoids translates to circadian control of EGFR signalling: EGFR signals are suppressed by high glucocorticoids during the active phase (night-time in rodents), while EGFR signals are enhanced during the resting phase. Consistent with this pattern, treatment of animals bearing EGFR-driven tumours with a specific kinase inhibitor was more effective if administered during the resting phase of the day, when glucocorticoids are low. These findings support a circadian clock-based paradigm in cancer therapy.
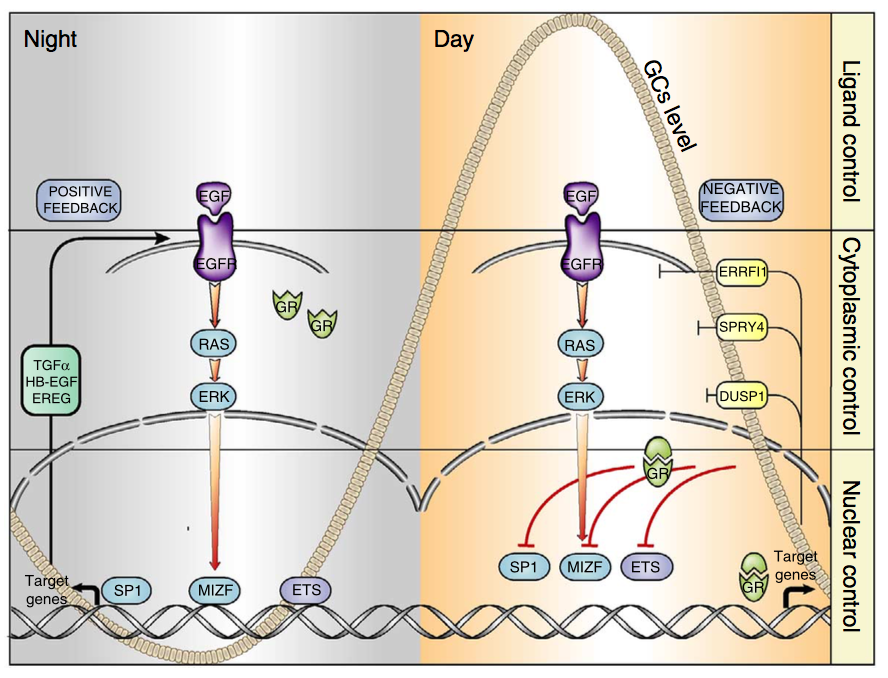
Go to the full article: Mattia Lauriola, Yehoshua Enuka, Amit Zeisel, Gabriele D’Uva, Lee Roth, Michal Sharon-Sevilla, Moshit Lindzen, Kirti Sharma, Nava Nevo, Morris Feldman, Silvia Carvalho, Hadas Cohen-Dvashi, Merav Kedmi, Nir Ben-Chetrit, Alon Chen, Rossella Solmi, Stefan Wiemann, Fernando Schmitt, Eytan Domany & Yosef Yarden. Diurnal suppression of EGFR signalling by glucocorticoids and implications for tumour progression and treatment. Nature Communications, 2015
06.10.2014 – Science Daily Tumors might grow faster at nigh
06.10.2014 – NATURE WORLD NEWS: Is Cancer Growth Nocturnal?
06.10.2014 – Softpedia: Cancer Tumors Appear to Grow Faster and Spread More Easily at Night
06.10.2014 – Medical Xpress: Tumors might grow faster at night: Hormone that keeps us alert also suppresses the spread of cancer
06.10.2014 – Senior Journal: Cancer Grows at Night, Maybe That’s When to Attack, New Study Says
07.10.2014 – Daily Mail: Cancerous tumours ‘grow faster at night’ – and drugs to fight the disease might work better during sleep, study finds
07.10.2014 – Counsel&Heal: Cancer Treatment More Efficient During Night Time:Study
07.10.2014 – Science 2.0: Cancer Might Grow Faster At Night
07.10.2014 – The Health Site: Revealed — cancer spreads during nights
07.10.2014 – bhataramedia: Tumor Dapat Tumbuh Lebih Cepat Di Malam Hari
07.10.2014 – Jews News: Israeli study: Tumors might grow more quickly at night
08.10.2014 – The Times of Israel: Tumors may grow faster at night, Israeli study shows
08.10.2014 – Journal de la Science: Les tumeurs grossiraient plus vite la nuit
08.10.2014 – Haaretz: Night time may be the right time to treat cancer, find Israeli scientists
09.10.2014 – Weizmann USA: Why Cancer Drugs May Work Better While You Sleep
09.10.2014 – Nature子刊:夜间癌细胞扩散的更快?
09.10.2014 – TIME: Why Cancer Drugs May Work Better While You Sleep
09-10-2014 – Medisite: CANCER : LES MÉDICAMENTS PLUS EFFICACES LA NUIT
10.10.2014 – TopSante: Cancer : les tumeurs se développent plus vite la nuit
10.10.2014 – Forbes: Cancer May Grow Faster While We Sleep
10.10.2014 – Jerusalem Post Tumors may grow faster at night, say Weizmann scientists
10.10.2014 – Medcenter: Una hormona que nos mantiene alerta también suprime la diseminación del cáncer
16.10.2014 – EACR (European Association for Cancer Research): TUMOURS MIGHT GROW FASTER AT NIGHT
16.10.2014 – Revista Genetica Medica: Ritmos circadianos y tratamiento contra el cáncer
17.10.2014 – University Herald: Hormone Active During Day Supresses Growth of Cancer Cells, Study
20.10.2014 – Stato Quotidiano: Ricercatrice Manfredonia: “tumori crescono più in fretta di notte”
24.10.2014 – Huffington Post: Does Cancer Grow More Aggressively at Night?
Hypoxia has been long-time acknowledged as major cancer-promoting microenvironment. In such an energy-restrictive condition, post-transcriptional mechanisms gain importance over the energy-expensive gene transcription machinery. Here we show that the onset of hypoxia-induced cancer stem cell features requires the beta-catenin-dependent post-transcriptional up-regulation of CA9 and SNAI2 gene expression. In response to hypoxia, beta-catenin moves from the plasma membrane to the cytoplasm where it binds and stabilizes SNAI2 and CA9 mRNAs, in cooperation with the mRNA stabilizing protein HuR. We also provide evidence that the post-transcriptional activity of cytoplasmic beta-catenin operates under normoxia in basal-like/triple-negative breast cancer cells, where the beta-catenin knockdown suppresses the stem cell phenotype in vitro and tumor growth in vivo. In such cells, we unravel the generalized involvement of the beta-catenin-driven machinery in the stabilization of EGF-induced mRNAs, including the cancer stem cell regulator IL6. Our study highlights the crucial role of post-transcriptional mechanisms in the maintenance/acquisition of cancer stem cell features and suggests that the hindrance of cytoplasmic beta-catenin function may represent an unprecedented strategy for targeting breast cancer stem/basal-like cells.

Go to the full article: Gabriele D’Uva*, Sara Bertoni, Mattia Lauriola, Sabrina De Carolis, Annalisa Pacilli, Laura D’Anello, Donatella Santini, Mario Taffurelli, Claudio Ceccarelli, Yosef Yarden, Lorenzo Montanaro, Massimiliano Bonafé, Gianluca Storci. Beta-Catenin/HuR Post-Transcriptional Machinery Governs Cancer Stem Cell Features in Response to Hypoxia. PloS One, 2013 (co-corresponding author)
We were selected for the oral presentation of our project entitled “Heart regeneration and tumorigenicity: oncogenic ErbB2 as a driving force” at EMBO meeting -“Cardiac Biology: From Development to Regenerative Medicine” (7-10 Jun 2013, EMBL Heidelberg, Germany).



The role of corticosterone (Cort), the immune system’s major stress hormone, in the regulation of hematopoietic stem and progenitor cells (HSPCs) and their dynamic bone marrow (BM) microenvironment is currently unknown. We report that corticotropin-releasing factor receptor 1 (CRFR1) mutant mice with chronically low Cort levels showed aberrant HSPC regulation, having higher HSPC numbers and upregulation of the chemokine CXCL12, phenotypes that were restored by Cort supplementation. Expanded stromal progenitors known to support HSPCs were also observed in these low-Cort-containing mice. A similar phenotype was induced in wild-type (WT) mice by Metyrapone, a Cort synthesis inhibitor. Conversely, high Cort exposure induced HSPC apoptosis, reduced long-term BM repopulation and decreased stromal progenitor cell numbers. We documented circadian oscillations of Cort in WT BM but not in CRFR1 mutant mice, leading to diminished circadian BM CXCL12 fluctuations and increased number of circulating HSPCs in these mice. Finally, low Cort induced expansion of stromal progenitors, CXCL12 expression, HSPC proliferation and BM repopulation capacity, involving Notch1 signaling. This was associated with upregulation of the Notch ligand, Jagged1, in BM myeloid cells. Our results suggest that daily physiologic Cort oscillations are critical for balanced HSPC proliferation and function involving Notch1 signaling and their supportive BM microenvironment.
Go to the full article: O Kollet*, Y Vagima*, G D’Uva, K Golan, J Canaani, T Itkin, S Gur-Cohen, A Kalinkovich, G Caglio, C Medaglia, A Ludin, K Lapid, E Shezen, A Neufeld-Cohen, D Varol, A Chen, T Lapidot. Physiologic corticosterone oscillations regulate murine hematopoietic stem/progenitor cell proliferation and CXCL12 expression by bone marrow stromal progenitors. Leukemia, 2013 (* equal contribution)
Regulation of hematopoietic stem and progenitor cell (HSPC) steady-state egress from the bone marrow (BM) to the circulation is poorly understood. While glycogen synthase kinase-3β (GSK3β) is known to participate in HSPC proliferation, we revealed an unexpected role in the preferential regulation of CXCL12-induced migration and steady-state egress of murine HSPCs, including long-term repopulating HSCs, over mature leukocytes. HSPC egress, regulated by circadian rhythms of CXCL12 and CXCR4 levels, correlated with dynamic expression of GSK3β in the BM. Nevertheless, GSK3β signaling was CXCL12/CXCR4 independent, suggesting that synchronization of both pathways is required for HSPC motility. Chemotaxis of HSPCs expressing higher levels of GSK3β compared with mature cells was selectively enhanced by stem cell factor-induced activation of GSK3β. Moreover, HSPC motility was regulated by norepinephrine and insulin-like growth factor-1 (IGF-1), which increased or reduced, respectively, GSK3β expression in BM HSPCs and their subsequent egress. Mechanistically, GSK3β signaling promoted preferential HSPC migration by regulating actin rearrangement and microtubuli turnover, including CXCL12-induced actin polarization and polymerization. Our study identifies a previously unknown role for GSK3β in physiological HSPC motility, dictating an active, rather than a passive, nature for homeostatic egress from the BM reservoir to the blood circulation.
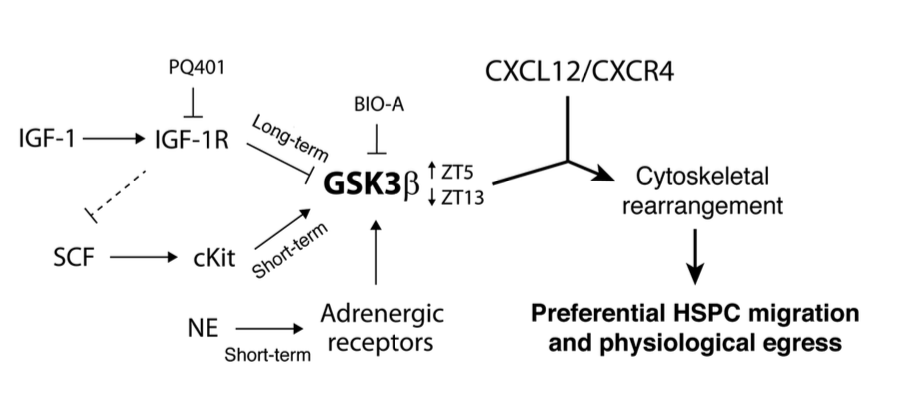
Go to the full article: Kfir Lapid, Tomer Itkin, Gabriele D’Uva, Yossi Ovadya, Aya Ludin, Giulia Caglio, Alexander Kalinkovich, Karin Golan, Ziv Porat, Massimo Zollo, Tsvee Lapidot. GSK3β regulates physiological migration of stem/progenitor cells via cytoskeletal rearrangement. The Journal of clinical investigation, 2013
 We won the poster session at the conference ISCS “The 4th Young Investigators Stem Cell Meeting” organized by the Israeli Stem Cell Society (December 2, 2012. Tel Aviv, Israel).
We won the poster session at the conference ISCS “The 4th Young Investigators Stem Cell Meeting” organized by the Israeli Stem Cell Society (December 2, 2012. Tel Aviv, Israel).
Presented project: D’Uva G, Lauriola M, Rajchman D, Weisinger K, Yarden Y and Tzahor E. “Heart regeneration and tumorigenicity: oncogenic ERBB2 as a driving force”.