The murine neonatal heart can regenerate after injury through cardiomyocyte (CM) proliferation, although this capacity markedly diminishes after the first week of life. Neuregulin-1 ( NRG1) administration has been proposed as a strategy to promote cardiac regeneration. Here, using loss- and gain-of-function genetic tools, we explore the role of the NRG1 co-receptor ERBB2 in cardiac regeneration. NRG1-induced CM proliferation diminished one week after birth owing to a reduction in ERBB2 expression. CM-specific Erbb2 knockout revealed that ERBB2 is required for CM proliferation at embryonic/neonatal stages. Induction of a constitutively active ERBB2 (ca ERBB2) in neonatal, juvenile and adult CMs resulted in cardiomegaly, characterized by extensive CM hypertrophy, dedifferentiation and proliferation, differentially mediated by ERK, AKT and GSK3β/ β-catenin signalling pathways. Transient induction of ca ERBB2 following myocardial infarction triggered CM dedifferentiation and proliferation followed by redifferentiation and regeneration. Thus, ERBB2 is both necessary for CM proliferation and sufficient to reactivate postnatal CM proliferative and regenerative potentials.
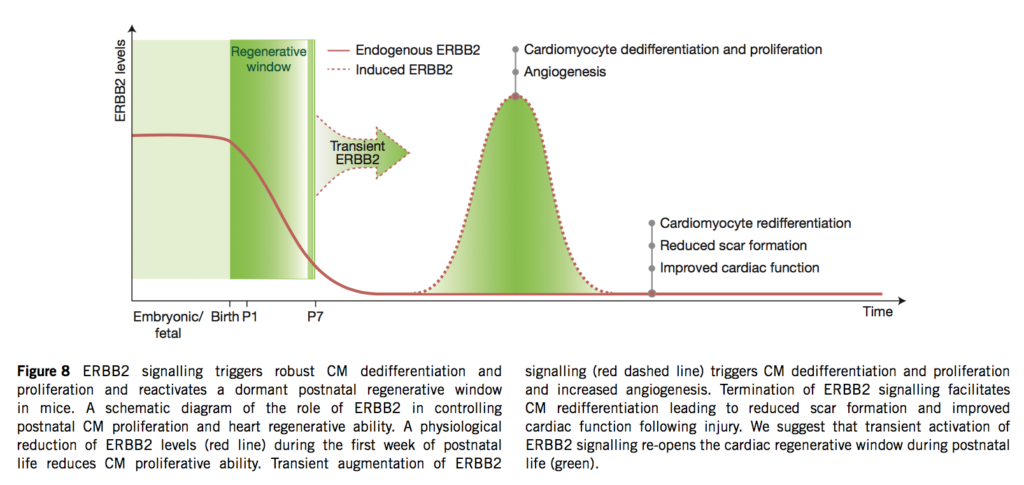
Go to the full article: Gabriele D’Uva, Alla Aharonov, Mattia Lauriola, David Kain, Yfat Yahalom-Ronen, Silvia Carvalho, Karen Weisinger, Elad Bassat, Dana Rajchman, Oren Yifa, Marina Lysenko, Tal Konfino, Julius Hegesh, Ori Brenner, Michal Neeman, Yosef Yarden, Jonathan Leor, Rachel Sarig, Richard P Harvey and Eldad Tzahor. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nature Cell Biology, 2015
Scientific recognitions
Science – News: New hope for heart attack sufferers?
NATURE – News and Views: Regenerative biology: Neuregulin 1 makes heart muscle
NATURE REVIEWS DRUG DISCOVERY – Research Highlight: Heart disease – Stimulating cardiomyocyte regeneration after heart failure
NABR: The Essential Need for Animals in Medical Research
MEDIA COVERAGE
06.04.2015 – The Guardian: Heart muscle cells regrown in medical research breakthrough
06.04.2015 – The Sydney Morning Herald: Australian researchers help find way to regrow heart muscle
07.04.2015 – RT: Scientists discover revolutionary method to regrow heart muscles
07.04.2015 – AM: Helping the heart repair itself after a cardiac arrest: researchers say they’ve worked out how
07.04.2015 – Financial Express: The Turbo-charging hormone can repair ‘broken’ heart
07.04.2015 – El Comercio: Logran regenerar músculos del corazón al estimular una hormona
07.04.2015 – SKY NEWS: Breakthrough Sees Heart Muscle Cells Regrown
07.04.2015 – SBS: Scientific breakthrough could give new hope to heart-attack patients
07.04.2015 – The Jewish Press: Israeli-Australian Researchers Discover How to Regrow Heart Muscle
07.04.2015 – Business Magazin: STUDIU: Cercetătorii au reuşit să stimuleze regenerarea celulelor muşchiului cardiac
07.04.2015 – iarul de Iasi: Cercetătorii au reuşit să stimuleze regenerarea celulelor muşchiului cardiac
07.04.2015 – ZeeNews: Scientists regenerate cardiac muscles through hormone stimulation
07.04.2015 – Medical Xpress: Research effort leads to mammalian heart tissue regeneration
07.04.2015 – Kurier: Durchbruch: Forscher lassen Herzmuskelzellen wachsen
08.04.2015 – SBS World NEWS: Researchers Trigger Heart Regeneration After Heart Attack (TV news)
08.04.2015 – The Times of Israel: Medical leap as Israeli researchers regrow heart cells
08.04.2015 – smarTherapy: Consiguen regenerar los músculos del corazón mediante estimulación hormonal
08.04.2015 – Scimondo: Aktivierung eines Hormon-Signalweges hilft Mäuseherzen nach einem Herzinfarkt auf die Sprünge
08.04.2015 – Beyond the Dish: Dead Heart Muscle Regrown in Rodents
08.04.2015 – Phys.org: Research finds turbo-charging hormone can regrow the heart
08.04.2015 – Newsmax: Hormone Found to Regenerate Heart Muscle
09.04.2015 – Medical Daily: Heart Attack Patients May Regrow Cardiac Cells By 2020 Thanks To Breakthrough Discovery
09.04.2015 – The Australian: Bashing corporate Australia has reunited the faceless man with his Green frenemies
10.04.2015 – SILICONWADI: Ricercatori israeliani fanno ricrescere le cellule del cuore
10.04.2015 – Italiasalute.it: Nuova tecnica per curare il cuore infartuato
10.04.2015 – Tiscali: Cuore infartuato, scoperto il meccanismo che avvia la formazione di nuove cellule
10.04.2015 – Ansa: Scoperta la chiave per riparare il cuore infartuato
10.04.2015 – MeteoWeb.eu: Salute: scoperta la chiave per riparare il cuore infartuato
10.04.2015 – Bionn: 拯救心脏 拒绝梗死! – 大健康产业专区- 生物谷
11.04.2015 – Giornale di Sicilia: Cuore colpito da infarto, scoperta la chiave per ripararlo
12.04.2015 – Il Quotidiano di Puglia: Con questo gene riparerò il cuore
12.04.2015 – NurseTimes: Scoperta la chiave per riparare il tessuto cardiaco infartuato
13.04.2015 – Il Sole 24 Ore: Cuore, identificato gene capace di “ripararlo”
13.04.2015 – HealthCanal: Heart Cells Regenerated in Mice
13.04.2015 – IBA: הלב את מחדשים
13.04.2015 – Radio Jai: Científicos de Israel regeneran células del corazón de ratones
13.04.2015 – EnlaceJudío: Científicos israelíes regeneran células cardíacas
13.04.2015 – israel21c: Weizmann Institute scientists regenerate heart cells in mice
13.04.2015 – IsraCast: Medical Breakthrough – Israeli Scientists Find Way to Regrow Heart Muscle Cells
13.04.2015 – Science Daily: Heart cells regenerated in mice
13.04.2015 – Popular Science: Scoperta la chiave per riparare il cuore infartuato
13.04.2015 – Science Codex: Heart cells regenerated in mice in NGR1 study
13.04.2015 – Science 2.0: Heart Cells Regenerated By Going Backward To Make Progress
13.04.2015 – ScienceBlogs – The Weizmann Wave: Guest post: Dr. Gabriele D’Uva: How to Grow New Heart Cells
13.04.2015 – Il Resto del Carlino: Nuove cellule grazie alla ricerca. Così si ripara il cuore infartuato
14.04.2015 – The Jerusalem Post: Weizmann Institute researchers regenerate heart cells in mice
14.04.2015 – Farmacia.it: Lotta all’infarto: il cuore potrà essere riparato
14.04.2015 – ANI News: Regeneration of heart cells possible in mice
14.04.2015 – Business Standard: Regeneration of heart cells possible in mice
14.04.2015 – New Kerala: Regeneration of heart cells possible in mice
14.04.2015 – Corriere del mezzogiorno: Studio il cuore, sogno l’Italia
14.04.2015 – Israel Hayom: Israeli scientists make revolutionary discovery, regenerate heart cells
14.04.2015 – Genetic Engeneering & Biotechnology News: Old Heart Cells Divide Like New
14.04.2015 – Daily News & Analysis: Scientists regenerate heart cells in mice
14.04.2015 – Cardiovascular Disease News: Study Advances Understanding of Heart Cells’ Regeneration
14.04.2015 – Comité Central Israelita Uruguay: Científicos israelíes regeneran células cardíacas
14.04.2015 – Prahova: Cercetatorii au reusit sa stimuleze regenerarea celulelor muschiului cardiac
14.04.2015 – Iran Daily: Heart cells regenerated in mice
14.04.2015 – Horizon 2020 projects: ERC-funded research sees mouse heart cells renewed
14.04.2015 – Israeli Ministry of Foreign Affairs: Heart cells regenerated in mice
14.04.2015 – MedIndia: Regeneration of Heart Cells Possible in Mice With the Help of Specialized Protein, ERBB2
14.04.2015 – DAILYROUNDS: Successful heart muscle regeneration in mice may soon be seen in humans
14.04.2015 – Jns: Israeli Scientists Regenerate Heart Cells in Revolutionary Discovery
15.04.2015 – The Algemeiner: Israeli Scientists Regenerate Heart Cells in Revolutionary Discovery
15.04.2015 – Nature子刊:ERBB2触发哺乳动物心脏再生是通过促进心肌细胞去分化和增殖
15.04.2015 – Sanità Salento: Un cuore rigenerato per gli infartuati (con intervista radiofonica)
15.04.2015 – Medical Insider: Ученые научились восстанавливать сердечную мышцу
16.04.2015 – Diario del web: Scienziati rigenerano le cellule del cuore
17.04.2015 – Innovations Report: Weizmann Institute Scientists Regenerate Heart Cells in Mice
18.04.2015 – Globus Magazine: RIGENERAZIONE CARDIACA: TROVATA LA CHIAVE PER RIPARARE IL CUORE INFARTUATO
19.04.2015 – Ultime Tecno-scientifiche: COSI’ SI RIPARA IL CUORE INFARTUATO
19.04.2015 – Corriere di Bologna: Gabriele, il ricercatore che ha riacceso il cuore e vuol tornare in Italia
20.04.2015 – Giannella channel: È italiano il medico che ha scoperto come riparare un cuore infranto
20.04.2015 – PolskieRadio: Komórki serca potrafią się zregenerować po zawale
21.04.2015 – multibriefs: Researchers regenerate heart cells in mice
23.04.2015 – UNIBO MAGAZINES: Ecco il gene chiave per riparare il cuore dopo un infarto
23.04.2015 – UNI news24: ERBB2: scoperto il gene che rigenera il cuore dopo un infarto
23.04.2015 – La Repubblica: Trovato un gene per riparare il cuore dopo un infarto
23.04.2015 – RaiNews: Un gene ripara-cuore
23.04.2015 – Rai3 – TGR Emilia Romagna (TV news – min. 15:34)
23.04.2015 – Bologna2000.it: Identificato un gene chiave capace di riparare il cuore danneggiato da un infarto
23.04.2015 – SassuoloOnline: Identificato un gene chiave capace di riparare il cuore danneggiato da un infarto
23.04.2015 – L’Adige: Scoperto gene per riparare il cuore dopo l’infarto
23.04.2015 – Controcampus.it: Gene chiave salva il cuore dopo un infarto, ricerca Unibo
23.04.2015 – Virgilio Notizie: Scoperto un gene per riparare il cuore dopo un infarto
23.04.2015 – TRC: Post infarto, importante scoperta medica
23.04.2015 – AGI: Ricerca Unibo: identificato gene per riparare cuore dopo infarto
23.04.2015 – BolognaToday: Ricerca Unibo: scoperto gene chiave per riparare il cuore dopo un infarto
27.04.2015 – Viversani: Scoperto un gene per riparare il cuore dopo un infarto
27.04.2015 – ResearchItaly: Identified the gene that can repair the heart after a heart attack
27.04.2015 – Biotechin: A “Hearty” discovery – Turbo-charging hormone can regrow the heart
28.04.2015 – Salzburger Nachrichten: Krebs-Wachstumsrezeptor steuert Regeneration von Herzzellen
29.04.2015 – HealthCanal: HEART ATTACK BREAKTHROUGH
02.05.2015 – LiveUniversity: Scoperto gene chiave capace di riparare il cuore danneggiato da un infarto
06.05.2015 – The Australian Jewish News: A heartening collaboration
06.05.2015 – Iton Gadol: Researchers Regenerate Heart Cells In What Could Be A Huge Breakthrough For Heart Disease Treatments
06.05.2015 – AJN: Avances. Investigadores israelíes regeneran células del corazón para tratar la enfermedad cardiac
06.05.2015 – NoCamels: Researchers Regenerate Heart Cells In What Could Be A Huge Breakthrough For Heart Disease Treatments
06.05.2015 – vetscite.org: Heart cells regenerated in mice
07.05.2015 – Yad be Yad: Investigadores israelíes regeneran células del corazón para tratar enfermedades cardíacas
08.05.2015 – Classic Chaos: Researchers Regenerate Heart Cells In What Could Be A Huge Breakthrough For Heart Disease Treatments
14.05.2015 – VitAssistance: Cuore, identificato gene capace di ripararlo
6.06.2015 – Ruthfully yours: Amazing Israel :Researchers Regenerate Heart Cells In What Could Be A Huge Breakthrough For Heart Disease Treatments


 We were selected for the oral presentation of our project entitled “ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation” at
We were selected for the oral presentation of our project entitled “ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation” at