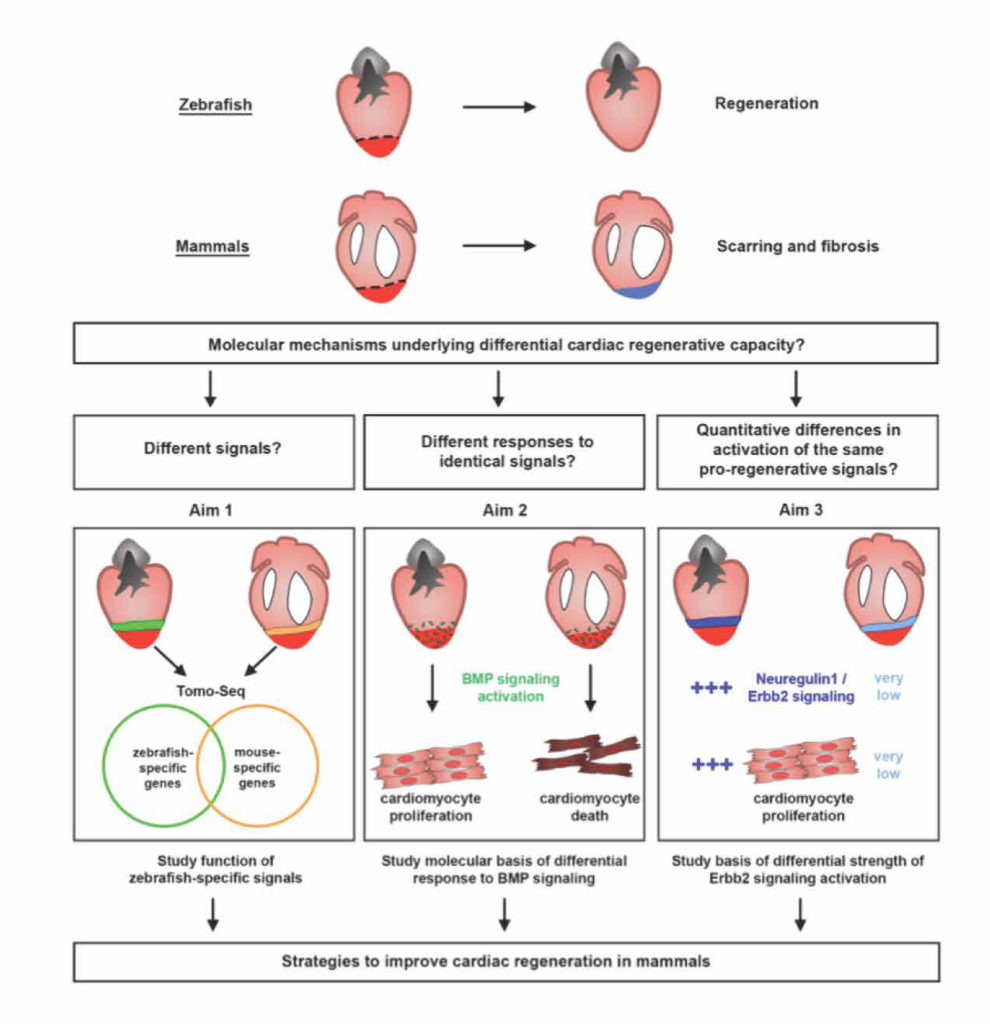
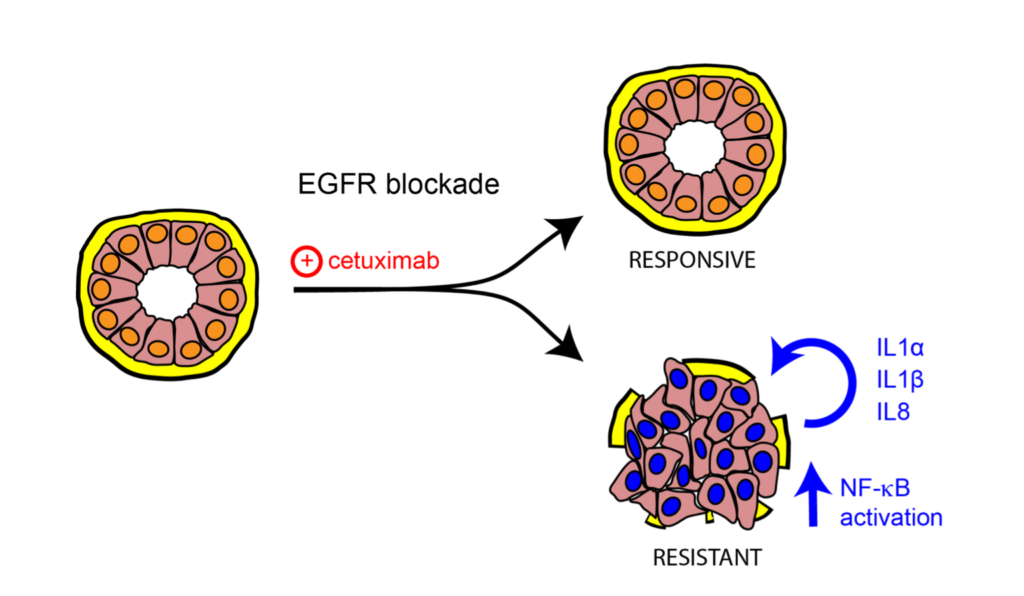
Epidermal Growth Factor Receptor (EGFR) activates a robust signalling network to which colon cancer tumours often become addicted. Cetuximab, one of the monoclonal antibodies targeting this pathway, is employed to treat patients with colorectal cancer. However, many patients are intrinsically refractory to this treatment, and those who respond develop secondary resistance along time. Mechanisms of cancer cell resistance include either acquisition of new mutations or non genomic activation of alternative signalling routes. In this study, we employed a colon cancer model to assess potential mechanisms driving resistance to cetuximab. Resistant cells displayed increased ability to grow in suspension as colonspheres and this phenotype was associated with poorly organized structures. Factors secreted from resistant cells were causally involved in sustaining resistance, indeed administration to parental cells of conditioned medium collected from resistant cells was sufficient to reduce cetuximab efficacy. Among secreted factors, we report herein that a signature of inflammatory cytokines, including IL1A, IL1B and IL8, which are produced following EGFR pathway activation, was associated with the acquisition of an unresponsive phenotype to cetuximab in vitro. This signature correlated with lack of response to EGFR targeting also in patient-derived tumour xenografts. Collectively, these results highlight the contribution of inflammatory cytokines to reduced sensitivity to EGFR blockade and suggest that inhibition of this panel of cytokines in combination with cetuximab might yield an effective treatment strategy for CRC patients refractory to anti-EGFR targeting.
Go to the full article: Gelfo V, Rodia MT, Pucci M, Dall’Ora M, Santi S, Solmi R, Roth L, Lindzen M, Bonafè M, Bertotti A, Caramelli E, Lollini PL, Trusolino L, Yarden Y, D’Uva G* and Lauriola M. A module of inflammatory cytokines defines resistance of colorectal cancer to EGFR inhibitors. Oncotarget 2016 (*co-last author)
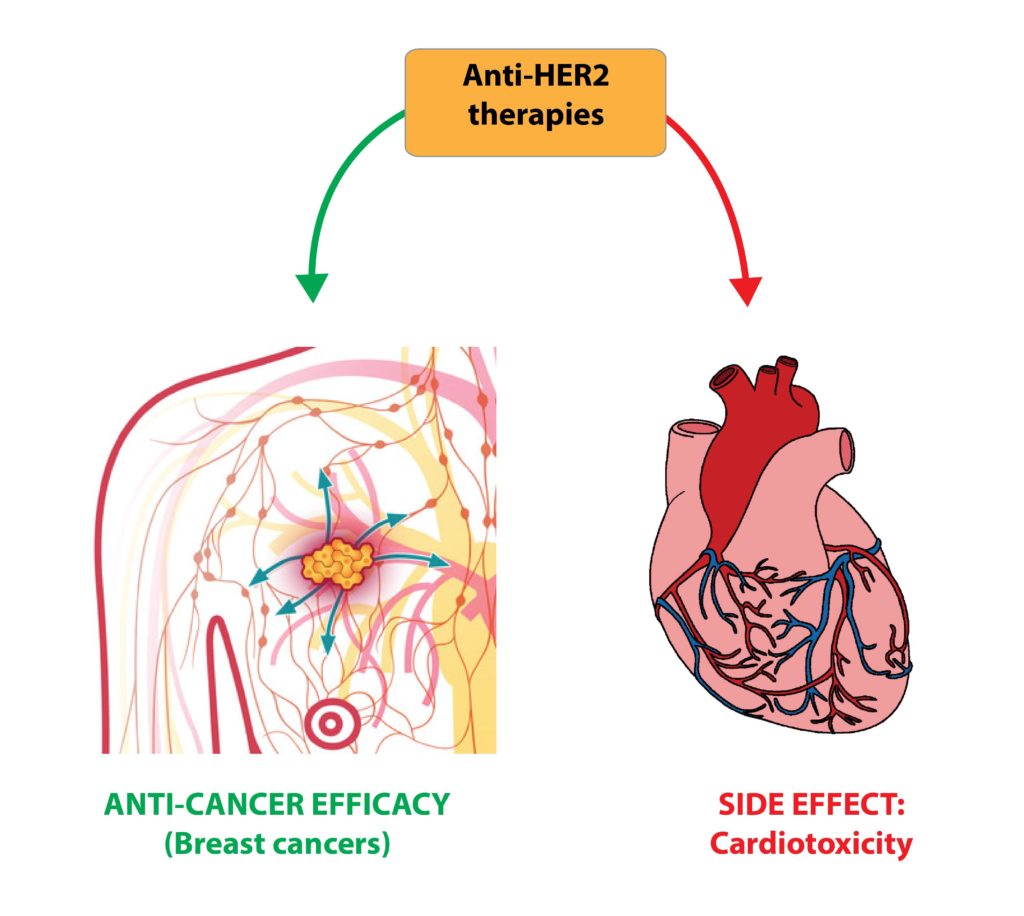

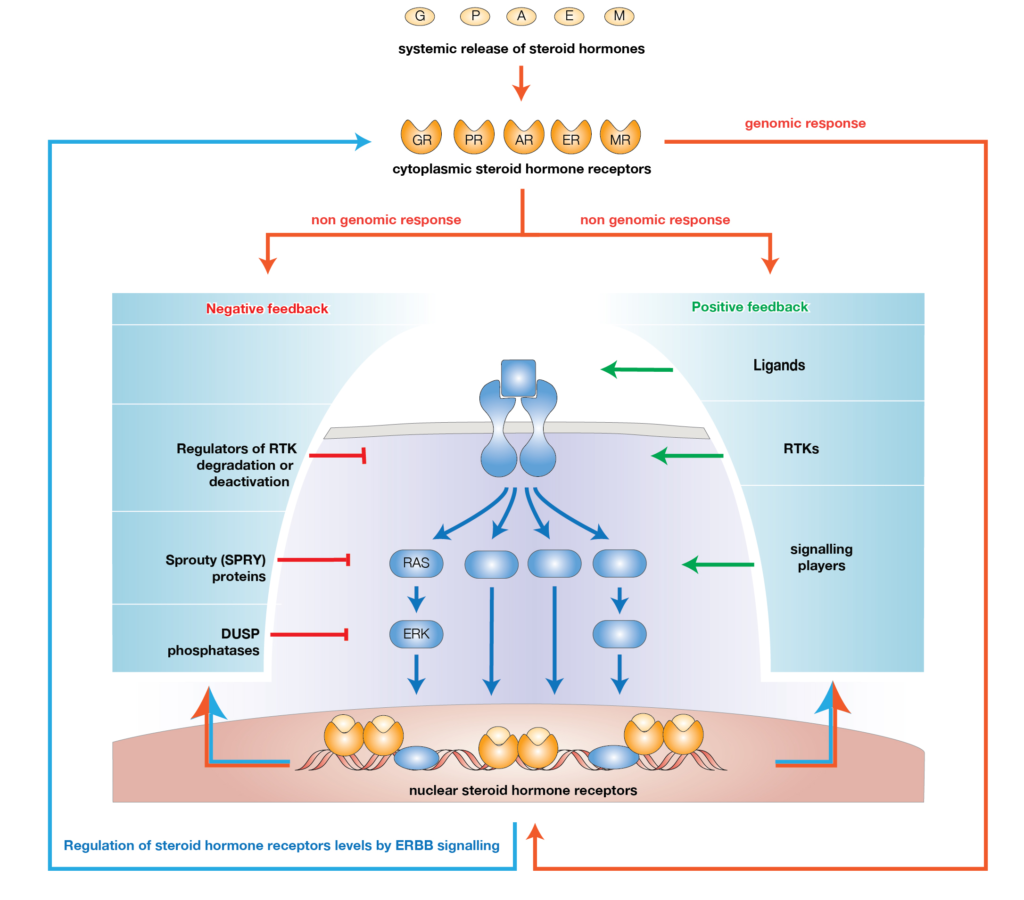
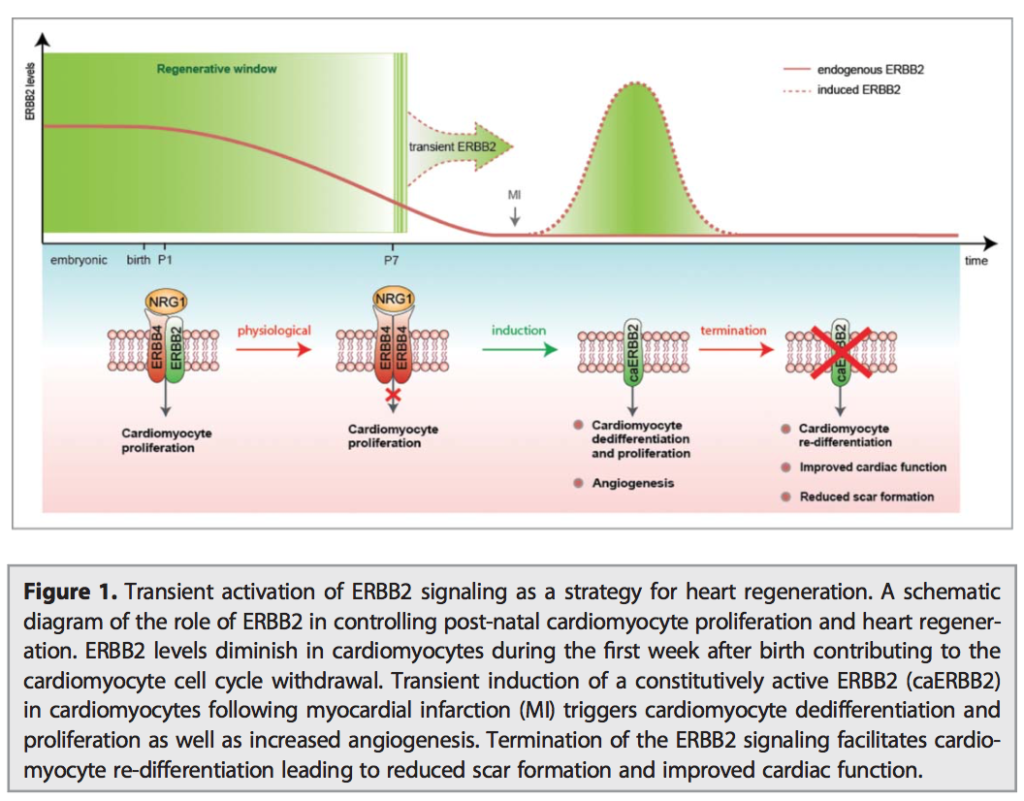
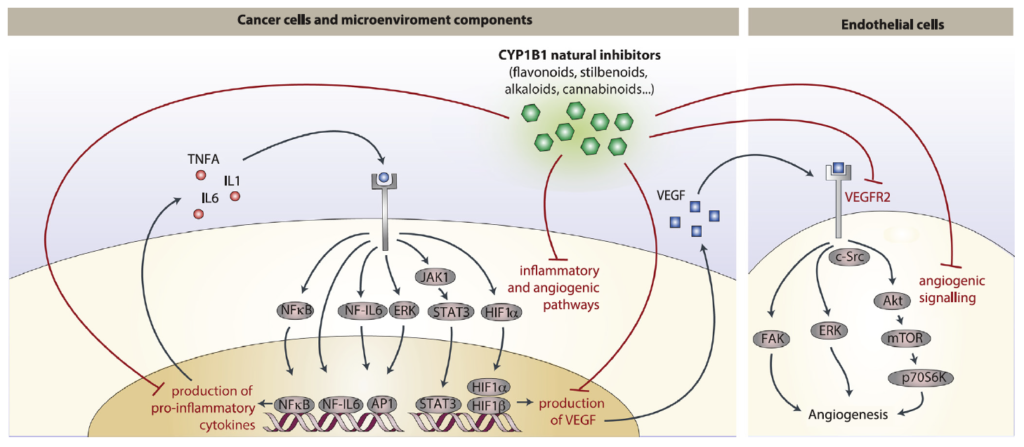 Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.
Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.