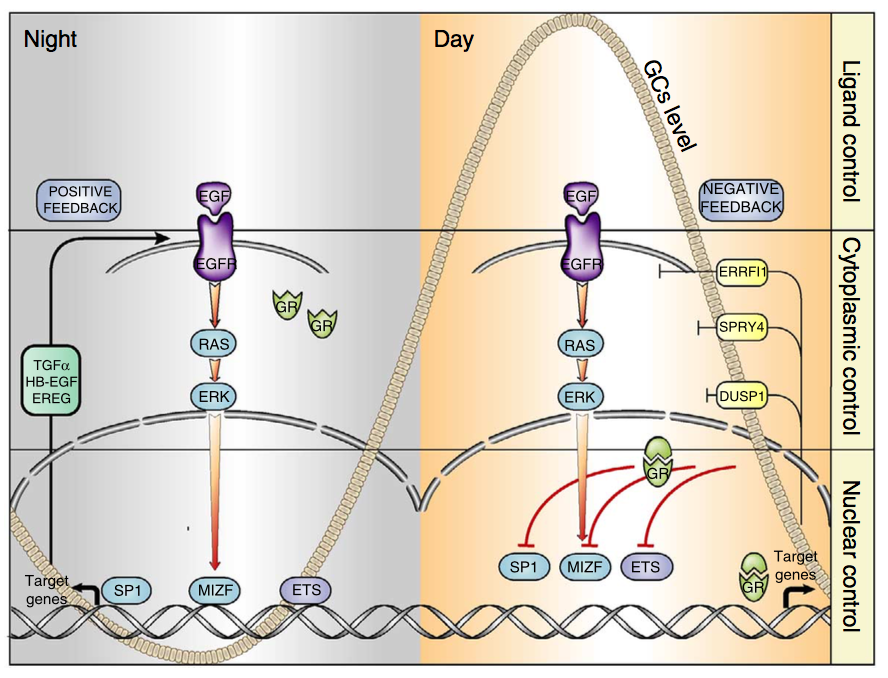
Breast cancers called “triple negative” and “basal-like” are particularly aggressive subtypes of breast cancer, with fewer treatment options.
Our results, just published in the journal “Cancers“, suggest that in these tumor subtypes the activation of a cellular signaling pathway composed by the growth factor Neuregulin and the ERBB3 and ERBB2 receptors may confer to cancer cells an increased ability to grow in absence of adhesion, consequently supporting the dissemination of tumor cells and the growth of metastases.
Therefore, our study suggests to evaluate, through further experiments, the administration of Neuregulin-ERBB3-ERBB2 axis inhibitors, in particular anti-ERBB2 drugs already used in the clinic for the treatment of other tumor subtypes, as a potential therapeutic strategy for basal-like / triple-negative breast cancer patients .
Congratulations to Dr. Carmen Miano, postdoc researcher of our research group and first author of the article, as well as to the other members of our group and external collaborators who have helped and supported us in the execution of the experiments and in the advancement of the project.
We deeply thank #AIRC and #FondazioneCariplo for their financial support of the project.
Go to the full article: Miano C, Morselli A, Pontis F, Bongiovanni C, Sacchi F, Da Pra S, Romaniello D, Tassinari R, Sgarzi M, Pantano E, Ventura C, Lauriola M, D’Uva G. NRG1/ERBB3/ERBB2 Axis Triggers Anchorage-Independent Growth of Basal-like/Triple-Negative Breast Cancer Cells. Cancers, 2022

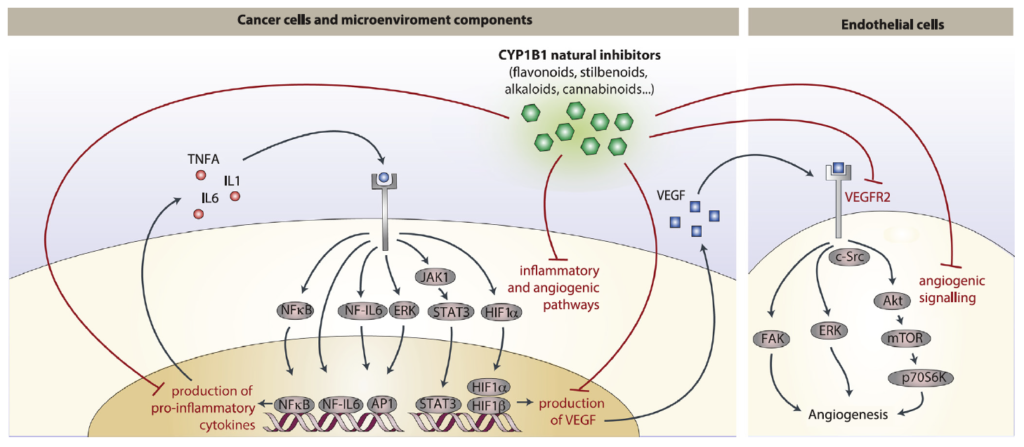 Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.
Abstract: Cancer chemoprevention is the use of synthetic, natural or biological agents to prevent or delay the development or progression of malignancies. Intriguingly, many phytochemicals with anti-inflammatory and anti-angiogenic effects, recently proposed as chemoprevention strategies, are inhibitors of Cytochrome P450 family 1B1 (CYP1B1), an enzyme overexpressed in a wide variety of tumors and associated with angiogenesis. In turn, pro-inflammatory cytokines were reported to boost CYP1B1 expression, suggesting a key role of CYP1B1 in a positive loop of inflammatory angiogenesis. Other well-known pro-tumorigenic activities of CYP1B1 rely on metabolic bioactivation of xenobiotics and steroid hormones into their carcinogenic derivatives. In contrast to initial in vitro observations, in vivo studies demonstrated a protecting role against cancer for the other CYP1 family members (CYP1A1 and CYP1A2), suggesting that the specificity of CYP1 family inhibitors should be carefully taken into account for developing potential chemoprevention strategies. Recent studies also proposed a role of CYP1B1 in multiple cell types found within the tumor microenvironment, including fibroblasts, endothelial and immune cells. Overall, our review of the current literature suggests a positive loop between inflammatory cytokines and CYP1B1, which in turn may play a key role in cancer angiogenesis, acting on both cancer cells and the tumor microenvironment. Strategies aiming at specific CYP1B1 inhibition in multiple cell types may translate into clinical chemoprevention and angioprevention approaches.