Ischemic heart diseases and cancers are leading causes of death worldwide. On one hand, cancer cells undergo deregulated proliferation. Conversely, the inability of cardiac muscle cells (cardiomyocytes) to re-enter into the cell cycle and proliferate is a major reason behind the lack of cardiac regenerative ability in adult mammals.
Our lab aims to understand molecular mechanisms driving cell differentiation and proliferation in these two diseases, in order to develop novel strategies to more effectively “block” cancer cells and to “unlock” the cardiac regenerative potential.
Furthermore, we are carrying out studies simultaneously focusing on tumour growth and heart function, in order to develop therapeutic strategies for the emerging problem of cardiotoxicity of anti-cancer therapies, which is responsible for a poor quality of life and reduced survival of cancer patients, regardless of the oncologic prognosis.
Cardiac regeneration
Severe cardiac injuries, such those induced by myocardial infarction (also known as heart attack), lead to a significant loss of cardiac muscle cells (cardiomyocytes), which are replaced by a non-contractile fibrotic scar at the infarct site. The loss of contractile cells and the inability of the adult mammalian heart to regenerate is a major cause of cardiac dysfunction and heart failure.
Stimulating the very low intrinsic proliferation rate of cardiomyocytes is a promising strategy for cardiac repair in patients with heart failure. It is therefore imperative to better understand the signalling pathways that promote cardiomyocyte proliferation.
We previously dissected important molecular mechanisms governing tissue renewal/regeneration in different tissues (Nature Cell Biology, 2015; Cell Cycle, 2015; Nature Immunology 2012; Nature Immunology 2011; JCI; 2013, Leukemia, 2013). In the cardiac tissue we demonstrated that the co-receptor ERBB2 is necessary for the proliferative response induced by the growth factor Neuregulin 1 (NRG1) during embryonic and neonatal stages. Further, we unveiled that cardiac ERBB2 levels decline soon after birth in mice, as part of the mechanism that leads to cardiomyocyte terminal differentiation and cell cycle withdrawal, and, consequently, loss of cardiac regenerative ability. By employing transgenic models, we demonstrated that a transient induction of ERBB2 signalling is sufficient to promote cardiomyocyte cell dedifferentiation and proliferation and to trigger a robust regenerative response following myocardial infarction in adult mice (Nature Cell Biology, 2015; Cell Cycle, 2015).
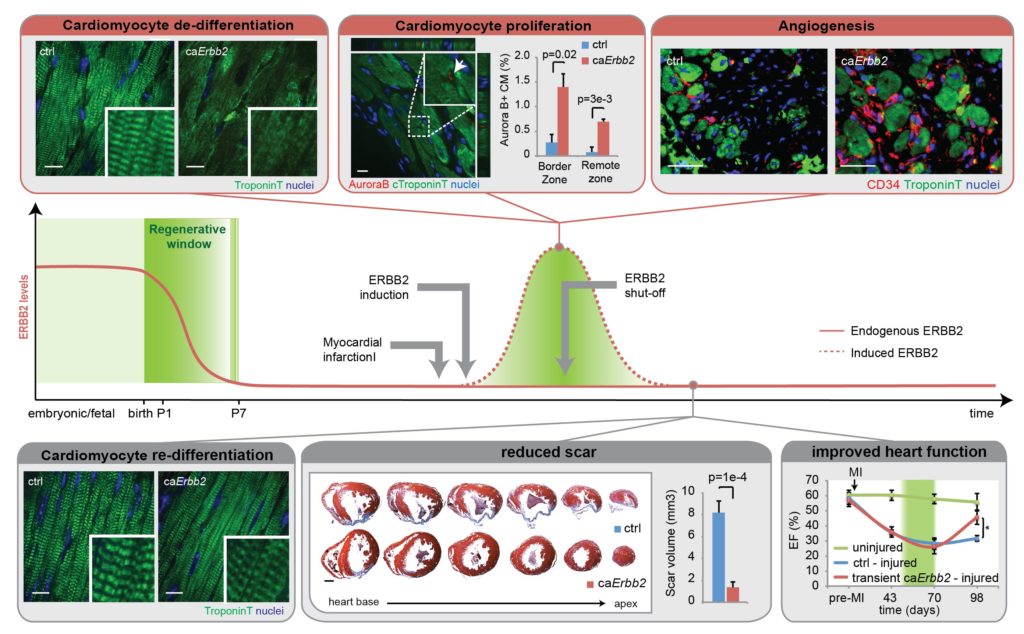 We are currently developing novel regenerative medicine approaches in the adult mammalian heart based on the stimulation of endogenous cardiomyocyte proliferation.
We are currently developing novel regenerative medicine approaches in the adult mammalian heart based on the stimulation of endogenous cardiomyocyte proliferation.
We thank the European Union’s Horizon 2020 research and innovation programme under the ERA-NET Co-fund action, Fondazione Fanti Melloni and Fondazione Carisbo and for supporting this research project.
Cancer & Cardiotoxicity
Cancer cell biology has played a crucial role in elucidating the molecular mechanisms by which oncogenic pathways sustain malignant behaviors and in identifying targets for anti-cancer drugs. Among them, growth factor receptors of ERBB family (composed of EGFR/ERBB1, ERBB2, ERBB3 and ERBB4) play major roles in a variety of cancer types. ERBB2 (also known as HER2) is the only ERBB receptor family member unable to bind ligands. However, it is the preferential partner for hetero-dimerization, powerfully enhancing and diversifying the downstream signaling pathway. Erbb2 is overexpressed in approximately 25-30% of breast carcinomas and in several other cancer types. These past decades have witnessed impressive advances in the comprehension of the driving role of the ERBB2 gene in cancer cell survival, proliferation, migration and resistance to chemotherapies. Pharmacological inhibition of ERBB2, via monoclonal antibodies or tyrosine kinase inhibitors, represents a milestone in cancer treatment. Unfortunately, cardio-toxicity is a serious side-effect of anti-HER2 therapies.
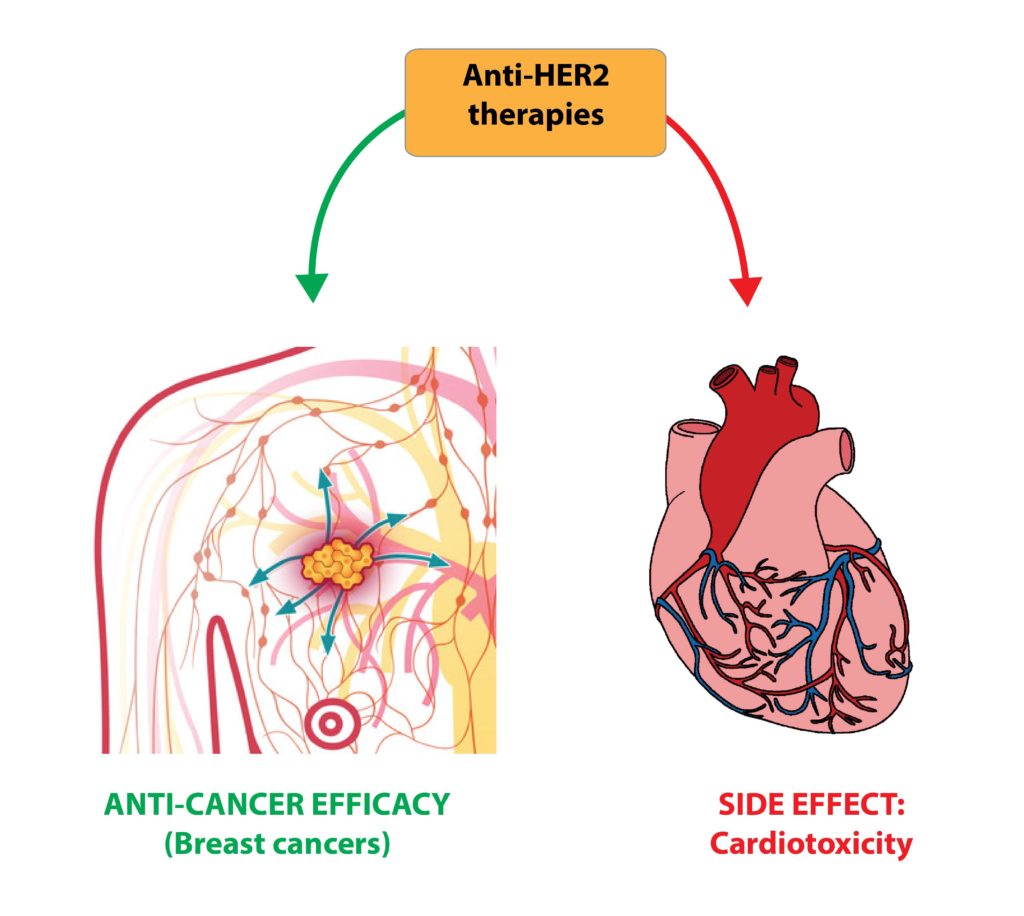
We previously contributed to dissecting the role, signaling and crosstalks of ERBB receptors in solid tumors (Nature Communication, 2014; Oncotarget 2016; Seminars in Cell and Developmental Biology, 2016; Plos One, 2013) as well as within the heart (Nature Cell Biology, 2015; Cell Cycle, 2015). By elucidating the molecular details of the ligand-receptor signaling axis NRGs-ERBBs in breast cancers and in the cardiac tissues, we aim at improving the efficacy and safety of HER2-targeted anti-cancer therapies.
We thank AIRC and Fondazione Cariplo for supporting this research project.